The only FDA-approved biologic and IL-17A antagonist for both JPsA and ERA1-8
For JPsA in children as young as 2 years and ERA in children as young as 4 years1
Here with you to give kids more time to be kids
Significantly longer time to flare in patients with JPsA1
In JUNIPERA in biologic-naive patients in Treatment Period 2
Time to flare in the individual JPsA subtype was an exploratory end point in Treatment Period 2. No clinical or statistical conclusions can be drawn.9
In JUNIPERA, the primary end point was time to flare in Treatment Period 2 in the combined JPsA and ERA patient population.9
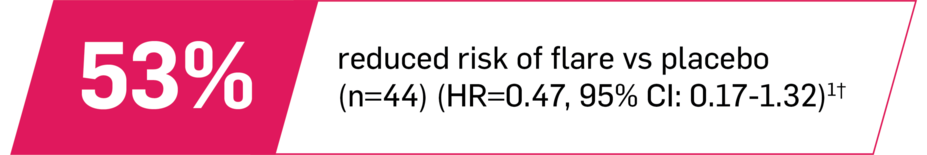
Longer time to flare in patients with ERA1
In JUNIPERA in biologic-naive patients in Treatment Period 2
Time to flare in ERA vs placebo (exploratory end point) did not reach statistical significance. Supplementary analyses provided confirmatory evidence of the treatment effect in ERA.1,9
In JUNIPERA, the primary end point was time to flare in Treatment Period 2 in the combined JPsA and ERA patient population.1
*During Treatment Period 2, a total of 11 patients with JPsA in the placebo group experienced a flare event compared with 4 patients with JPsA in the COSENTYX® group.1
†During Treatment Period 2, a total of 10 patients with ERA in the placebo group compared with 6 patients with ERA in the COSENTYX group.1
Definitions
ACR, American College of Rheumatology; ERA, enthesitis-related arthritis; FDA, US Food and Drug Administration; HR, hazard ratio; IL, interleukin; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis.
References
1. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
2. Humira. Prescribing information. AbbVie Inc.
3. Orencia. Prescribing information. Bristol-Myers Squibb Co.
4. Actemra. Prescribing information. Genentech Inc.
5. Ilaris. Prescribing information. Novartis Pharmaceuticals Corp.
6. Simponi Aria. Prescribing information. Janssen Biotech Inc.
7. Xeljanz. Prescribing information. Pfizer Inc.
8. Taltz. Prescribing information. Eli Lilly & Co.
9. Data on file. CAIN457F2304 (JUNIPERA): Clinical Study Report. Novartis Pharmaceuticals Corp; June 2020.