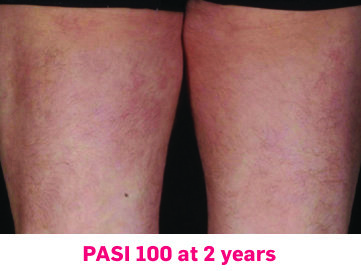
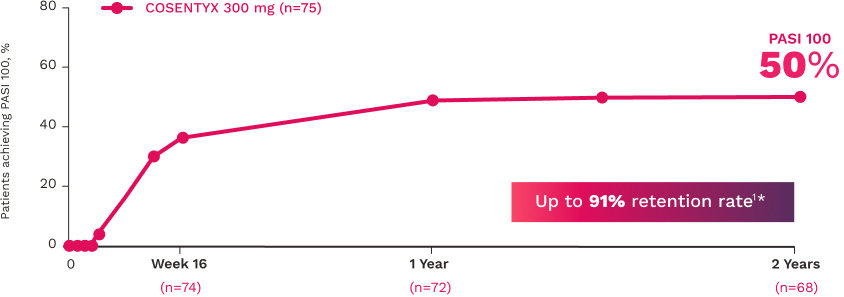
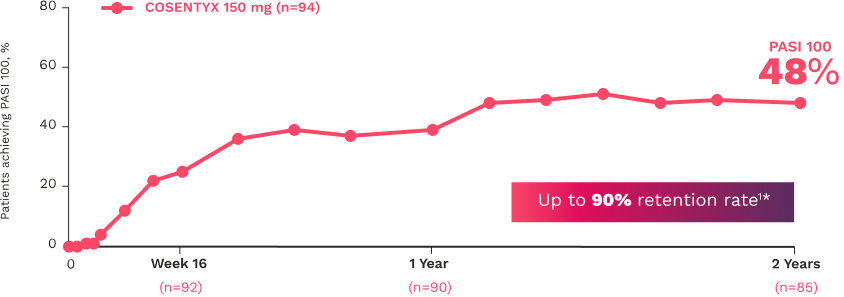
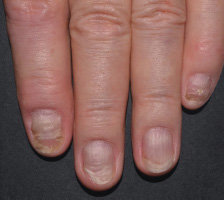
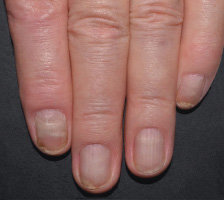
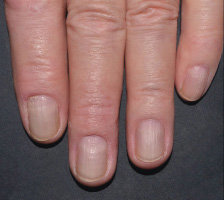
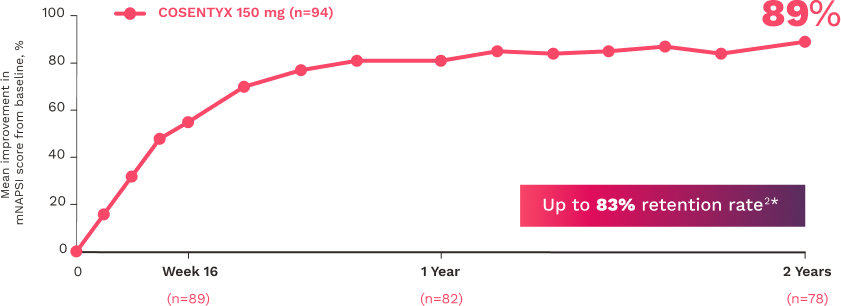
In FUTURE 5
COSENTYX clinical trial end points
FUTURE 2
FUTURE 2 studied a mixed population of patients with PsA: 2/3 biologic-naive and 1/3 anti–TNF-α inadequate responders15
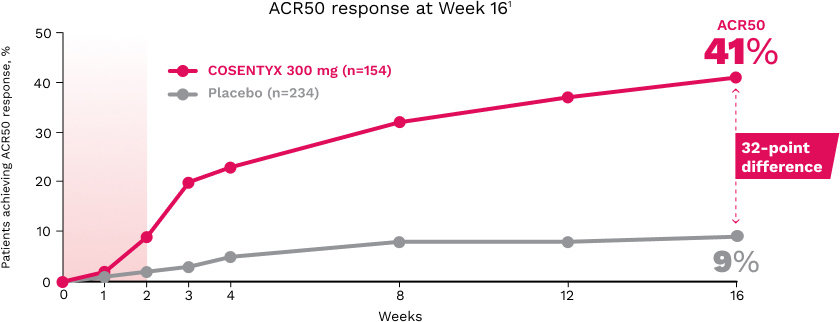
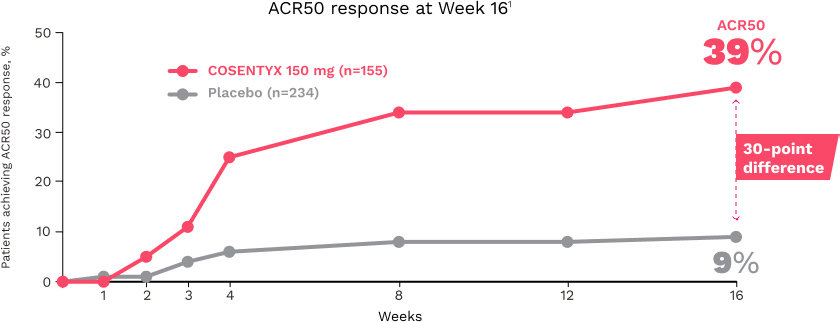
ACR20 responses at Week 16 (NRI) in the mixed population were 57% (P<0.0001), 60% (P<0.0001), and 18%, respectively, for COSENTYX 300 mg (n=100), 150 mg (n=100), and placebo (n=98)1,15
The primary end point of ACR20 responses at Week 24 (NRI) was achieved by 54% of patients on COSENTYX 300 mg (n=100) (P<0.0001), 51% on COSENTYX 150 mg (n=100) (P<0.0001), and 15% on placebo (n=98)1,15
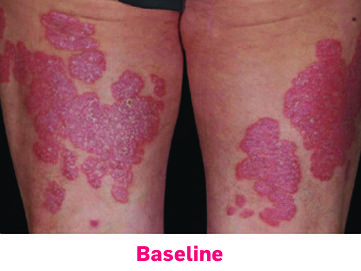
Skin lesions improved in patients with coexistent PsO who received COSENTYX (n=99) compared with placebo (n=43), as measured by the PASI1,15
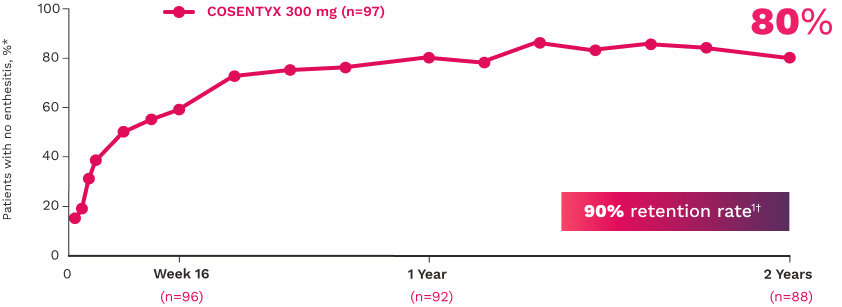
Improvements in enthesitis and dactylitis scores were observed in the COSENTYX group compared with placebo at Week 241
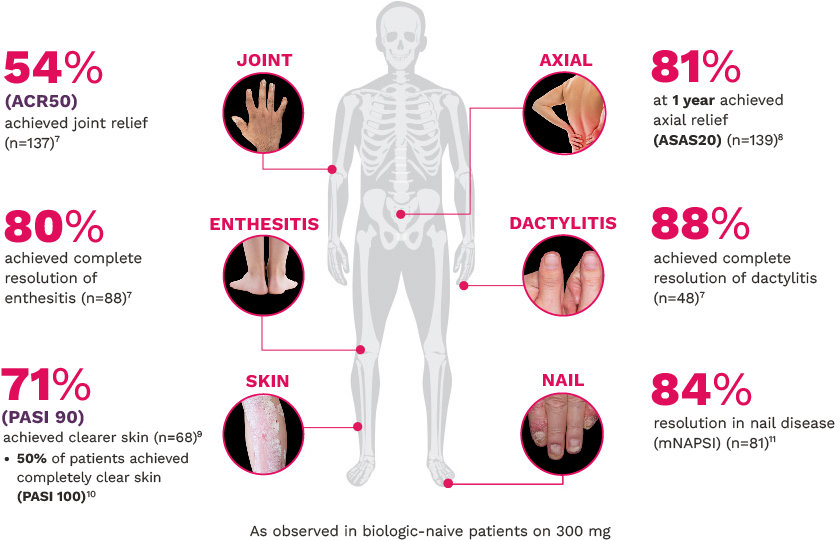
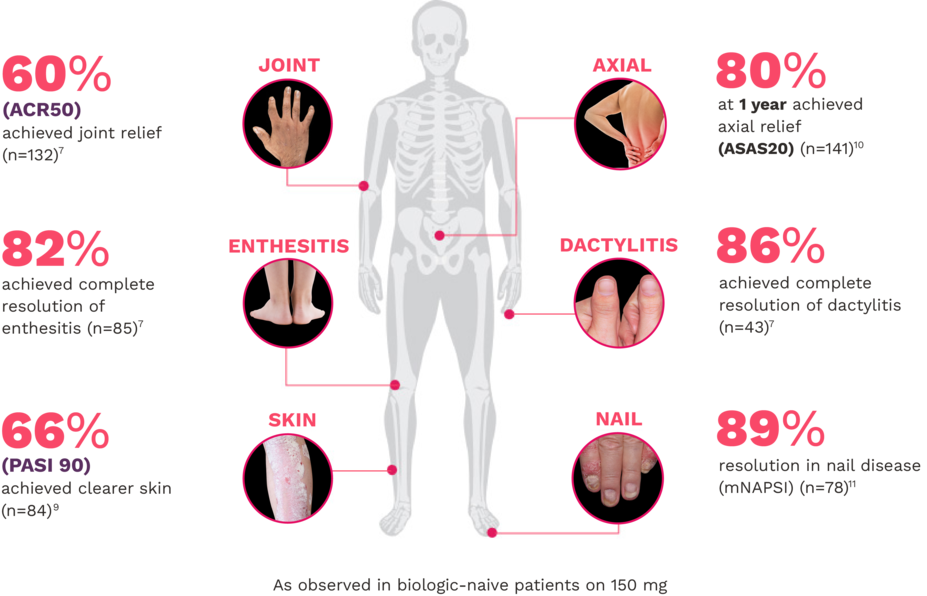
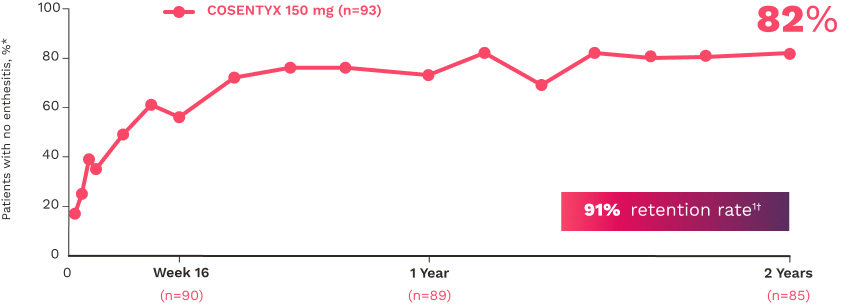
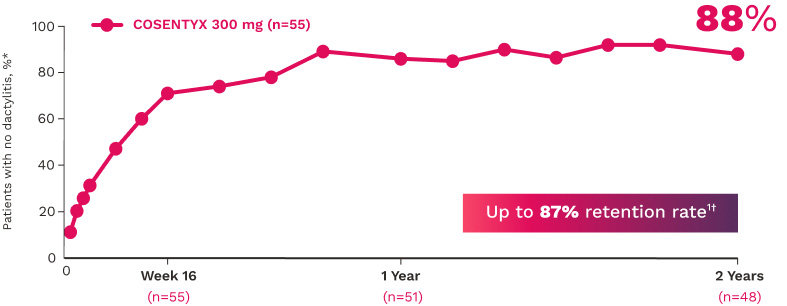
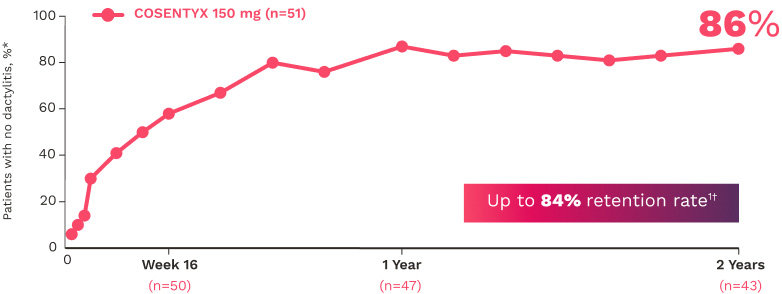
FUTURE 5
FUTURE 5 studied a mixed population of patients with PsA: more than 2/3 biologic-naive and less than 1/3 anti–TNF-α inadequate responders12
JOINT12
ACR20 response rates (NRI) (primary end point) were:
63% for COSENTYX 300 mg (n=222) (P<0.0001)
56% for COSENTYX 150 mg (n=220) (P<0.0001)
27% for placebo (n=332)
ENTHESITIS12
Complete resolution (LEI=0) in patients with enthesitis at baseline (NRI):
56% for COSENTYX 300 mg (n=140)
55% for COSENTYX 150 mg (n=141)
35% for placebo (n=192)
DACTYLITIS12
Complete resolution (LDI=0) in patients with dactylitis at baseline (NRI):
66% for COSENTYX 300 mg (n=82)
57% for COSENTYX 150 mg (n=80)
32% for placebo (n=124)
SKIN12
Patients who achieved PASI 90 (NRI):
54% for COSENTYX 300 mg (n=110)
37% for COSENTYX 150 mg (n=125)
9% for placebo (n=162)
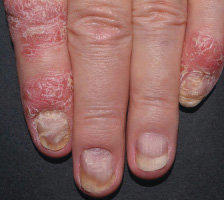
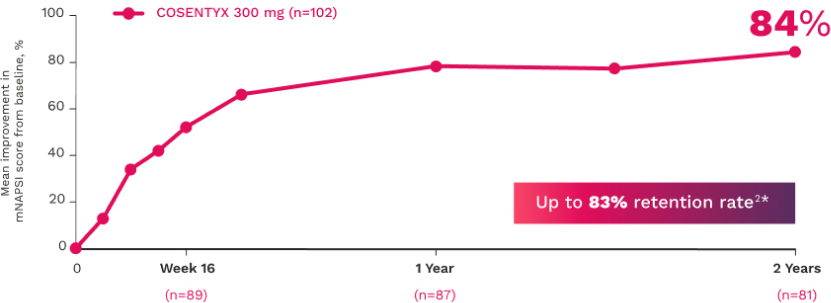
NAIL12*
Reduction in nail disease (mNAPSI as observed) response rates were†:
51% for COSENTYX 300 mg (n=125)
53% for COSENTYX 150 mg (n=128)
11% for placebo (n=203)
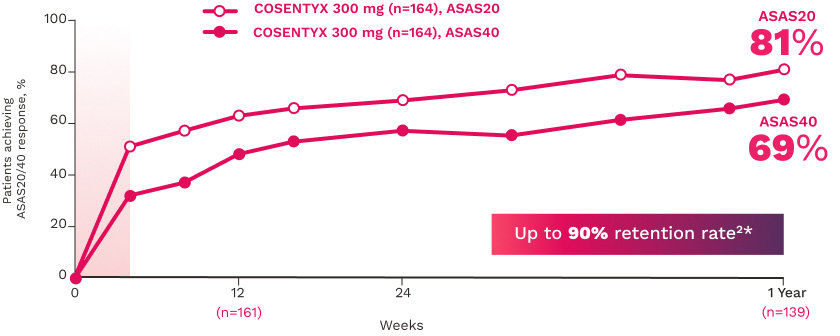
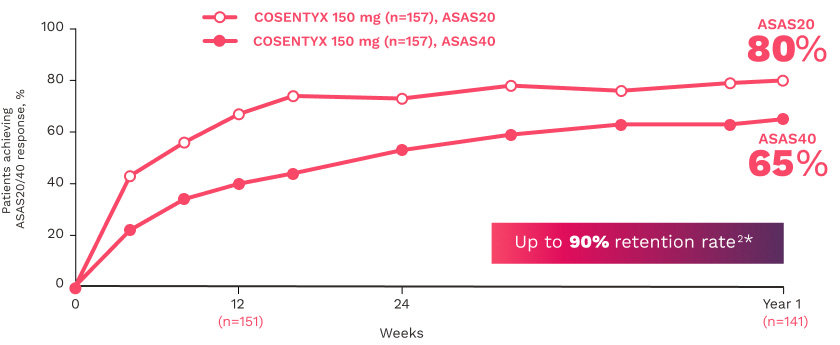
MAXIMISE
MAXIMISE studied biologic-naive patients with PsA16
The primary end point (ASAS20 responses at Week 12) was achieved by 63% of patients on COSENTYX 300 mg (n=164) compared with 31% for placebo (n=164) (MI; P<0.0001)16
The key secondary end point, ASAS20 responses at Week 12 in patients on COSENTYX 150 mg, was achieved by 66% (n=157) (MI; P<0.0001)16
TRANSFIGURE
TRANSFIGURE studied patients with nail psoriasis13,17
The primary end point, improvement from baseline NAPSI at Week 16 (repeated measures analysis), was 46.1% for COSENTYX 300 mg (n=63) and 11.7% for placebo (n=56) (P<0.0001)13,17
*Nail data in PsA were consistent with those in the dedicated nail PsO study, TRANSFIGURE. Improvement from baseline NAPSI at Week 16 (primary end point) (repeated measures analysis) was 46.1% for COSENTYX 300 mg (n=63) and 11.7% for placebo (n=56) (P<0.0001).13
†At baseline, mNAPSI scores were 18.0 and 17.8 in the 300 mg and 150 mg arms, respectively. At Week 16, mNAPSI scores were 8.8 and 8.4 in the 300 mg and 150 mg arms, respectively.12
Study designs
FUTURE 2 study design
FUTURE 2 was a multicenter, randomized, double-blind, placebo-controlled trial that evaluated 397 adult patients with active PsA (≥3 swollen and ≥3 tender joints) despite use of NSAIDs, corticosteroids, or DMARDs. Patients had a diagnosis for ≥5 years and received subcutaneous COSENTYX 75 mg (n=99), 150 mg (n=100), 300 mg (n=100), or placebo (n=98) at Weeks 0, 1, 2, 3, and 4, followed by the same dose every 4 weeks thereafter. Patients who received placebo were rerandomized to COSENTYX 150 mg or 300 mg every 4 weeks at Week 16 or Week 24 based on responder status. Primary end point was the percentage of patients with ACR20 response at Week 24. Study population was mixed: 2/3 of patients were biologic-naive and 1/3 were anti–TNF-α inadequate responders.1,15,18
FUTURE 5 study design
FUTURE 5 was a phase 3, multicenter, randomized, double-blind, placebo-controlled trial that evaluated 996 adult patients with active PsA. Patients were randomized to receive subcutaneous COSENTYX 150 mg without load (n=222), 150 mg with load (n=220), 300 mg with load (n=222), or placebo (n=332). Patients who received placebo were rerandomized to receive COSENTYX 150 mg or 300 mg every 4 weeks, based on responder status at Week 16 (nonresponders) or Week 24 (responders). Primary end point was the percentage of patients with ACR20 response at Week 16. Secondary end points included change in mTSS at Week 24 from baseline. Study population was mixed: more than 2/3 of patients were biologic-naive and less than 1/3 were anti–TNF-α inadequate responders (individual patients could have been exposed to up to 3 TNF-α inhibitors).12,19
MAXIMISE study design
MAXIMISE was a randomized, double-blind, placebo-controlled, multicenter, 52-week study that evaluated 498 patients with active PsA and axial skeleton involvement (defined by BASDAI score ≥4, spinal pain VAS ≥40 [0 to 100 mm scale]) who had inadequate response to at least 2 NSAIDs for at least 4 weeks. Patients were randomized to receive subcutaneous COSENTYX 150 mg (n=165), COSENTYX 300 mg (n=167), or placebo (n=166) at Weeks 0, 1, 2, 3, 4, and 8. Primary end point was the proportion of patients with ASAS20 response at Week 12. After Week 12, patients who were placed in the placebo group at baseline were rerandomized to active treatment with COSENTYX 150 mg or 300 mg, administered every 4 weeks from Week 12 to Week 52 (last dose on Week 48) but remained blinded to dose.16
TRANSFIGURE study design
TRANSFIGURE was a double-blind, randomized, placebo-controlled study examining the safety and efficacy of COSENTYX in patients with moderate to severe nail PsO. Patients were randomized to COSENTYX 300 mg (n=66), COSENTYX 150 mg (n=67), or placebo (n=65). All patients were adults with moderate to severe PsO (PASI score ≥12 and BSA ≥10%) and significant nail involvement (fingernail NAPSI score of ≥16 and ≥4 fingernails involved), who were candidates for systemic therapy. Primary end point: NAPSI assessment at Week 16. Secondary end points included NAPSI response over time up to Week 132.17
ACR, American College of Rheumatology; ASAS, Assessment of SpondyloArthritis international Society criteria; BASDAI, Bath Ankylosing Spondylitis Activity Index; BSA, body surface area; DMARDs, disease-modifying antirheumatic drugs; LDI, Leeds Dactylitis Index; LEI, Leeds Enthesitis Index; MI, multiple imputation; mNAPSI, modified Nail Psoriasis Severity Index; mTSS, modified Total Sharp Score; NAPSI, Nail Psoriasis Severity Index; NRI, nonresponder imputation; NSAIDs, nonsteroidal anti-inflammatory drugs; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, plaque psoriasis; TNF, tumor necrosis factor; VAS, visual analog scale.
Definitions
ACR, American College of Rheumatology; AS, ankylosing spondylitis; ASAS, Assessment of SpondyloArthritis international Society criteria; FDA, US Food and Drug Administration; IL, interleukin; IV, intravenous; MI, multiple imputation; mNAPSI, modified Nail Psoriasis Severity Index; NAPSI, Nail Psoriasis Severity Index; nr-axSpA, non-radiographic axial spondyloarthritis; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, plaque psoriasis; SC, subcutaneous.
References
Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
Remicade. Prescribing information. Janssen Biotech Inc.
Simponi Aria. Prescribing information. Janssen Biotech Inc.
Orencia. Prescribing information. Bristol-Myers Squibb Co.
Taltz. Prescribing information. Eli Lilly & Co.
Siliq. Prescribing information. Bausch Health US LLC.
Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report. Novartis Pharmaceuticals Corp; May 2019.
Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590.
Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report PASI 90 and ACR Components data. Novartis Pharmaceuticals Corp; January 2020.
Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report PASI 100 Tender/Swollen Joints Pain Biologic-Naive. Novartis Pharmaceuticals Corp; April 2020.
Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report mNAPSI and PASI 100 data. Novartis Pharmaceuticals Corp; October 2019.
Data on file. CAIN457F2342 Clinical Study Report Interim Analysis-Week 24. Novartis Pharmaceuticals Corp; November 2017.
Data on file. CAIN457A2313 Clinical Study Report. Novartis Pharmaceuticals Corp; November 2015.
Data on file. Novartis press release. Novartis Pharmaceuticals Corp; January 2016.
Data on file. CAIN457F2312 Clinical Study Report. Novartis Pharmaceuticals Corp; October 2014.
Data on file. CAIN457F3302 (MAXIMISE) Data Analysis Report. Novartis Pharmaceuticals Corp; June 2016.
Data on file. CAIN457A2313 Clinical Study Report. Novartis Pharmaceuticals Corp; October 2017.
Data on file. CAIN457F2312 Interim Study Report. Novartis Pharmaceuticals Corp; November 2015.
Mease P, van der Heijde D, Landewé R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77(6):890-897.
a. Baraliakos X, Gossec L, Pournara E, et al. Secukinumab in patients with psoriatic arthritis and axial manifestations: results from the double-blind, randomised, phase 3 MAXIMISE trial. Ann Rheum Dis. 2021;80(5):582-590.
b. Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report. Novartis Pharmaceuticals Corp; May 2019.
c. Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report PASI 90 and ACR Components data. Novartis Pharmaceuticals Corp; January 2020.
d. Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report mNAPSI and PASI 100 data. Novartis Pharmaceuticals Corp; October 2019.
e. Data on file. CAIN457H2315 Data Analysis Report. Novartis Pharmaceuticals Corp; April 2020.
f. Data on file. CAIN457H2315 Clinical Study Report. Novartis Pharmaceuticals Corp; November 2019.
g. Data on file. CAIN457F2310 Data Analysis Report. Novartis Pharmaceuticals Corp; June 2019.
h. Data on file. CAIN457F2310 (MEASURE 2): Nocturnal Back Pain. Novartis Pharmaceuticals Corp; February 2021.
i. Data on file. CAIN457F2342 (FUTURE 5): Interim Data Analysis Report FACIT-Fatigue data through Week 52. Novartis Pharmaceuticals Corp; April 2019.
j. Poddubnyy DA, Rudwaleit M, Listing J, Braun J, Sieper J. Comparison of a high sensitivity and standard C reactive protein measurement in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Ann Rheum Dis. 2010;69(7):1338-1341.
k. Data on file. CAIN457F2342 Clinical Study Report Interim Analysis-Week 24. Novartis Pharmaceuticals Corp; November 2017.
l. Data on file. CAIN457F2342 (FUTURE 5): 2-Year HAQ-DI biologic-naive data. Novartis Pharmaceuticals Corp; February 2021.
m. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
n. Data on file. Selected EAIRs MEASURE 2 Year 5. Novartis Pharmaceuticals Corp; January 2020.
o. Nash P, Mease PJ, McInnes IB, et al; on behalf of the FUTURE 3 study group. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20(1):47.
p. Data on file. LTD Cosentyx Prescriber and Patient Counts. Novartis Pharmaceuticals Corp; July 2021.
q. Boonen A, Sieper J, van der Heijde D, et al. The burden of non-radiographic axial spondyloarthritis. Semin Arthritis Rheum. 2015;44(5):556-562.
r. Data on file. CAIN457F2304 Clinical Study Report. Novartis Pharmaceuticals Corp; June 2020.
s. Data on file. CAIN457F2304 Data Analysis Report. Novartis Pharmaceuticals Corp; January 2022.
t. Bodemer C, Kaszuba A, Kingo K, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35(4):938-947.