Help your patients with PsA achieve remission
As defined by disease activity at 2 years1-4*
Please see clinical trial and primary end points, key data, and study designs
In FUTURE 5,† as observed in biologic-naive patients
150 mg at 2 years: 50% of patients achieved MDA (n=138)1
40% of biologic-naive patients in the COSENTYX 150-mg arm were up-titrated to 300 mg starting at Week 52, at the investigator’s discretion.1
In FUTURE 5, MDA was a prespecified exploratory end point at 2 years in a subgroup of biologic-naive patients. No clinical or statistical conclusions can be drawn.5
Reaching MDA involves achieving 5 of the following 7 criteria: ≤1 tender joint count; patient global assessment of disease activity VAS (≤20); ≤1 swollen joint count; tender entheseal points ≤1; PASI ≤1 or BSA ≤3%; patient pain VAS ≤15; HAQ-DI ≤0.5.5
All patients who met escape criteria (<20% improvement in tender or swollen joint counts) at Week 16 were considered nonresponders at Week 20 and Week 24. Results were analyzed using nonresponder imputation.6
*In addition to MDA, PsA disease activity may be assessed using DAS28, which is based on a count of 28 swollen and tender joints, patient global assessment of disease activity VAS, and either ESR or CRP level. A DAS28 score >5.1 implies active disease, ≤3.2 low disease activity, and <2.6 remission. EULAR response criteria are based on DAS28 status in combination with DAS28 improvements.2,6,9
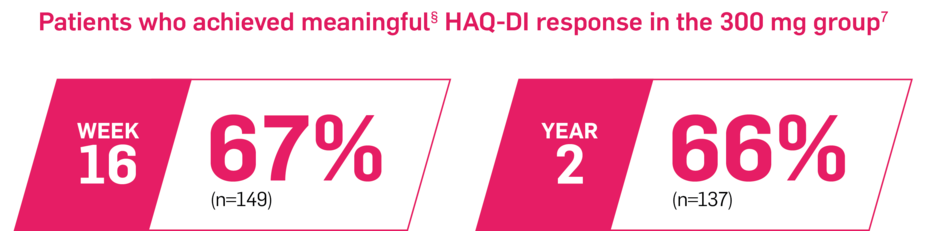
Majority of patients had improvements in physical functioning7
In FUTURE 5, as observed in biologic-naive patients7
150 mg at Week 16 and Year 2: 58% (n=148) and 66% (n=134) of patients achieved a meaningful HAQ-DI response, respectively (as observed)7
In FUTURE 5, HAQ-DI was a prespecified exploratory end point at Week 16 and 2 years in a subgroup of biologic-naive patients. No clinical or statistical conclusions can be drawn.5,7
The HAQ-DI score is an assessment of a patient’s functional ability to get dressed, get in and out of bed, eat, walk, perform hygiene activities, reach, grip and open doors, and perform daily activities. The HAQ-DI includes 20 questions in 8 categories of functioning.6
In FUTURE 5 at Week 16, in a mixed population (secondary end point): least-squares mean change from baseline HAQ-DI score was -0.55 and -0.44 in the COSENTYX 300-mg and 150-mg arms, respectively, and -0.21 in the placebo arm. At baseline, mean HAQ-DI scores were 1.2, 1.3, and 1.3 for the groups receiving COSENTYX 300 mg, 150 mg, and placebo, respectively.6
Help your patients manage their fatigue
In FUTURE 5, using MMRM analysis in biologic-naive patients8
At 1 year, patients in the 300-mg arm and the 150-mg arm experienced a 40% and 61% mean change from baseline in FACIT-Fatigue (n=147), respectively8
In FUTURE 5, fatigue was measured by FACIT-Fatigue, a prespecified exploratory end point at 1 year in a subgroup of biologic-naive patients. No clinical or statistical conclusions can be drawn.5,8
At 1 year in the mixed population, the FACIT-Fatigue scores were 14.1 and 16.9 for groups receiving COSENTYX 300 mg (n=206) and COSENTYX 150 mg (n=202), respectively.5,8
At baseline in the mixed population, the FACIT-Fatigue scores were 21.6, 23.6, and 21.7 for groups receiving COSENTYX 300 mg (n=220), COSENTYX 150 mg (n=218), and placebo (n=328), respectively. At Week 16, the FACIT-Fatigue scores were 15.4, 17.1, and 18.8 for groups receiving COSENTYX 300 mg (n=214), COSENTYX 150 mg (n=210), and placebo (n=303), respectively.5
The FACIT-Fatigue is a 13-item questionnaire that assesses self-reported fatigue and its impact on daily activities and function on a scale of 0 (“not at all”) to 4 (“very much”).5
‡58% and 50% of patients achieved MDA at 2 years on COSENTYX 300 mg (n=140) and COSENTYX 150 mg (n=138), respectively (as observed).1
§Defined as a change from baseline HAQ-DI score of ≥0.3.5
Definitions
BSA, body surface area; CRP, C-reactive protein; DAS, disease activity score; ESR, erythrocyte sedimentation rate; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire-Disability Index; MDA, minimal disease activity; MMRM, mixed-effect model repeated measure; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; SC, subcutaneous; VAS, visual analog scale.
References
1. Data on file. CAIN457F2342 (FUTURE 5): 2-Year Interim Report. Novartis Pharmaceuticals Corp; May 2019.
2. Lubrano E, Perrotta FM. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol. 2016;43(9):1765-1766.
3. Gossec L, Kerschbaumer A, Ferreira RJO, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2023 update. Ann Rheum Dis. 2024;83(6):706-719.
4. Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465-479.
5. Data on file. CAIN457F2342 (FUTURE 5): Clinical Study Report. Interim Analysis-Week 24. Novartis Pharmaceuticals Corp; November 2017.
6. Mease P, van der Heijde D, Landewé R, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77(6):890-897.
7. Data on file. CAIN457F2342 (FUTURE 5): 2-Year HAQ-DI biologic-naive data. Novartis Pharmaceuticals Corp; February 2021.
8. Data on file. CAIN457F2342 (FUTURE 5): Interim Data Analysis Report. FACIT-Fatigue data through Week 52. Novartis Pharmaceuticals Corp; April 2019.
9. Wells G, Becker J-C, Teng J, et al. Validation of the 28-joint Disease Activity Score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68(6):954-960.
10. Data on file. CAIN457F2312 (FUTURE 2): Clinical Study Report. Novartis Pharmaceuticals Corp; October 2014.
11. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
12. Data on file. CAIN457F2312 (FUTURE 2): Interim Study Report. Novartis Pharmaceuticals Corp; November 2015.
13. Data on file. CAIN457A2313 (TRANSFIGURE): Clinical Study Report. Novartis Pharmaceuticals Corp; November 2015.