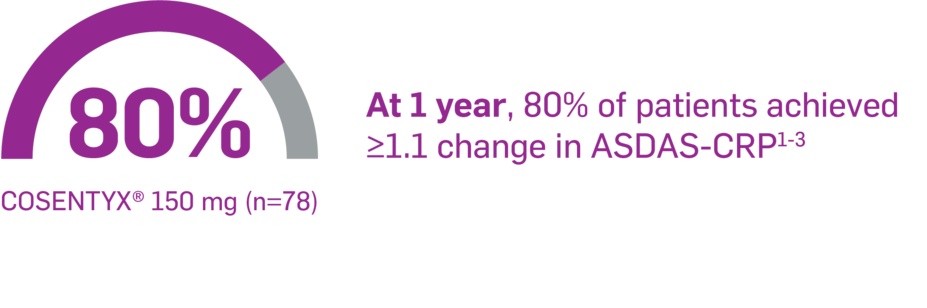Help your patients with nr-axSpA achieve LASTING disease control
Please see clinical trial and primary end points, key data, and study designs
In PREVENT,* as observed in a mixed population
At Week 16, 52% of patients achieved ≥1.1 change in ASDAS-CRP on COSENTYX 150 mg (n=176)3
In PREVENT, change in ASDAS-CRP ≥1.1 was an exploratory end point in a mixed population. No clinical or statistical conclusions can be drawn.2,3
ASAS-EULAR Guidelines define clinically important improvement as ≥1.1 change in ASDAS-CRP after at least 12 weeks of treatment.1
ASDAS-CRP is a composite index used to assess disease activity and includes CRP levels, patient global assessment, and BASDAI.4
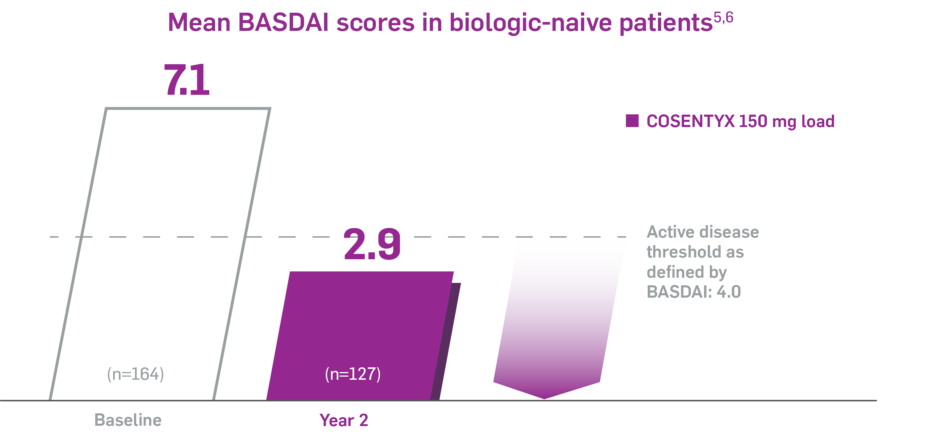
Reduction in overall disease activity with COSENTYX5
In PREVENT, as observed in biologic-naive patients
In PREVENT, BASDAI was an exploratory analysis as observed from baseline through Year 2. No clinical or statistical conclusions can be drawn.3
*Trial used SC administration.
Definitions
AS, ankylosing spondylitis; ASAS, Assessment of SpondyloArthritis International Society; ASDAS-CRP, Ankylosing Spondylitis Disease Activity Score–C-reactive protein; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; CRP, C-reactive protein; EULAR, European Alliance of Associations for Rheumatology; nr-axSpA, non-radiographic axial spondyloarthritis; SC, subcutaneous.
References
1. Ramiro S, Nikiphorou E, Sepriano A, et al. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann Rheum Dis. 2023;82(1):19-34.
2. Deodhar A, Blanco R, Dokoupilová E, et al. Improvement of signs and symptoms of nonradiographic axial spondyloarthritis in patients treated with secukinumab: primary results of a randomized, placebo-controlled phase III study. Arthritis Rheumatol. 2021;73(1):110-120.
3. Data on file. CAIN457H2315 (PREVENT): Clinical Study Report. Novartis Pharmaceuticals Corp; November 2019.
4. Data on file. CAIN457F2310 (MEASURE 2): Clinical Study Report. Novartis Pharmaceuticals Corp; November 2014.
5. Data on file. CAIN457H2315 (PREVENT): Data Analysis Report. Novartis Pharmaceuticals Corp; August 2021.
6. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.