Trust the efficacy and safety you know in the IV formulation your patients may need1*
For appropriate adult patients with PsA, AS, or nr-axSpA
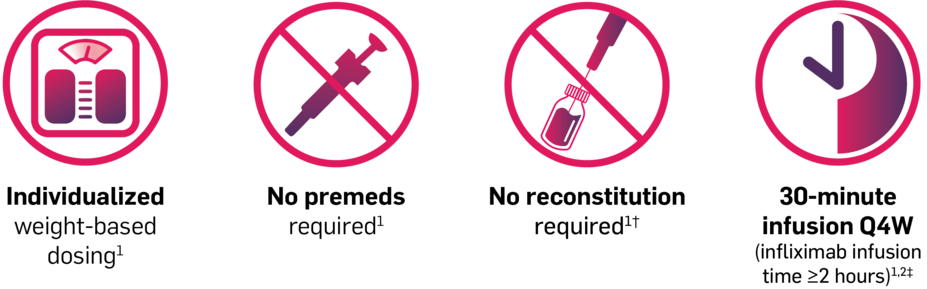
Recommended IV dosing schedule for adults with PsA, AS, or nr-axSpA1
COSENTYX® IV formulation can also be administered without a loading dose at 1.75 mg/kg every 4 weeks1
Total doses exceeding 300 mg per infusion are not recommended for the 1.75-mg/kg maintenance dose in patients with PsA, AS, or nr-axSpA1
*The effectiveness and safety of COSENTYX IV formulation are based on the pharmacokinetic exposure and extrapolation of the established effectiveness and safety of SC COSENTYX in adult patients with active PsA, AS, or nr-axSpA.1
†COSENTYX for IV use requires dilution prior to administration.1
‡This is not intended to compare the relative safety or efficacy of these treatments for PsA and AS. Please refer to the full Prescribing Information of each agent for dosage and administration.
Permanent J-code J32473
Definitions
AS, ankylosing spondylitis; IV, intravenous; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; Q4W, every 4 weeks; SC, subcutaneous.
References
1. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
2. Remicade. Prescribing information. Janssen Biotech Inc.
3. Centers for Medicare & Medicaid Services. Centers for Medicare & Medicaid Services (CMS) Healthcare Common Procedure Coding System (HCPCS) ApplicationSummaries and Coding Recommendations. First Quarter, 2024 HCPCS Coding Cycle. US Dept of Health and Human Services; 2024. Accessed April 11, 2024. https://www.cms.gov/files/document/2024-hcpcs-application-summary-quarter-1-2024-drugs-and-biologicals-posted-04/02/2024.pdf