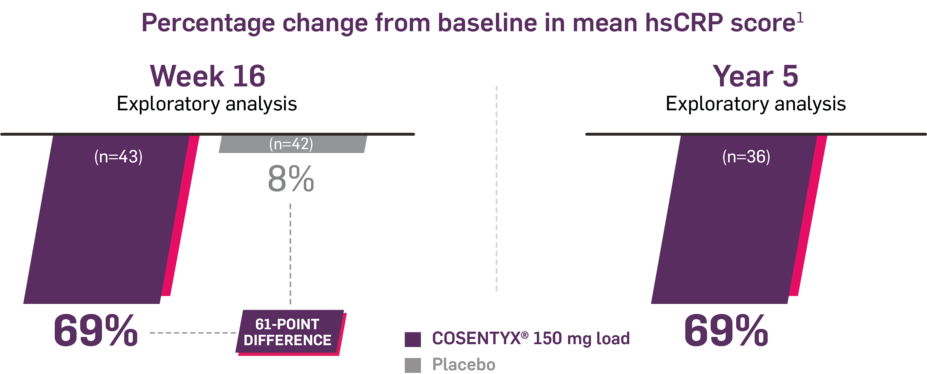
FAST and LASTING reductions in inflammation1
Please see clinical trial and primary end points, key data, and study designs
In MEASURE 2,* as observed in biologic-naive patients
hsCRP correlates better than routine CRP with clinical parameters in patients with AS2
In MEASURE 2, change in hsCRP was an exploratory end point at Week 16 and Year 5 in a subgroup of biologic-naive patients. No clinical or statistical conclusions can be drawn.1,3
Mean baseline hsCRP mg/L levels: 23.8 (150-mg group), 16.6 (placebo group). At Week 16, mean baseline hsCRP levels were 24.15 and 16.82 (COSENTYX 150-mg and placebo groups, respectively). At Week 16, mean change from baseline: -16.7, -1.4, respectively.1
At Year 5, mean baseline hsCRP level was 20.8 (COSENTYX 150 mg). At Year 5, mean change from baseline was -14.4 (150 mg).1
*Trial used SC administration.
Definitions
AS, ankylosing spondylitis; CRP, C-reactive protein; hsCRP, high-sensitivity C-reactive protein; SC, subcutaneous.
References
1. Data on file. CAIN457F2310 (MEASURE 2): Data Analysis Report. Novartis Pharmaceuticals Corp; June 2019.
2. Poddubnyy DA, Rudwaleit M, Listing J, Braun J, Sieper J. Comparison of a high sensitivity and standard C reactive protein measurement in patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Ann Rheum Dis. 2010;69(7):1338-1341.
3. Data on file. CAIN457F2310 (MEASURE 2): Clinical Study Report. Novartis Pharmaceuticals Corp; November 2014.
4. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.