For children with moderate to severe plaque psoriasis
Provide skin clearance with COSENTYX® (secukinumab)2
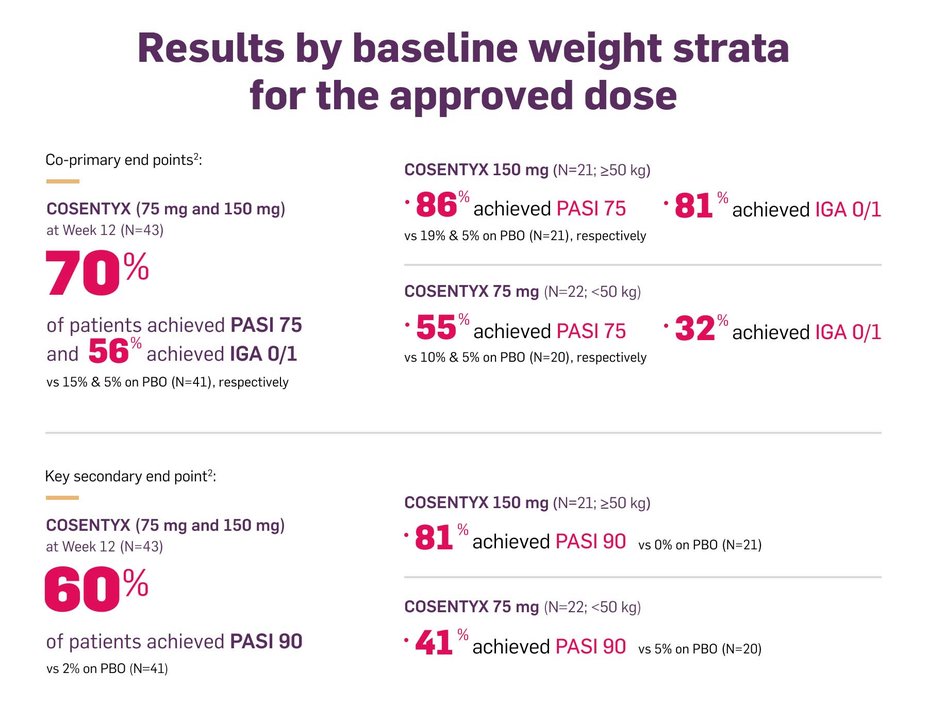
Pivotal Trial PsO6 in pediatric patients with severe PsO
Randomized, double-blind, PBO- and active-controlled trial to demonstrate efficacy and assess safety and tolerability of COSENTYX vs PBO and etanercept (in a single-blinded arm) in subjects from 6 to <18 years of age with severe chronic PsO (defined by PASI score ≥20, an IGA modified 2011 score of 4, and involving ≥10% of the BSA) who were candidates for systemic therapy. Nonresponder imputation (NRI) is used to handle missing values.2
Dosing of COSENTYX2: Patients <50 kg received 75 mg; patients ≥50 kg received 150 mg; administered by subcutaneous injection at Weeks 0, 1, 2, 3, and 4, followed by dosing every 4 weeks.
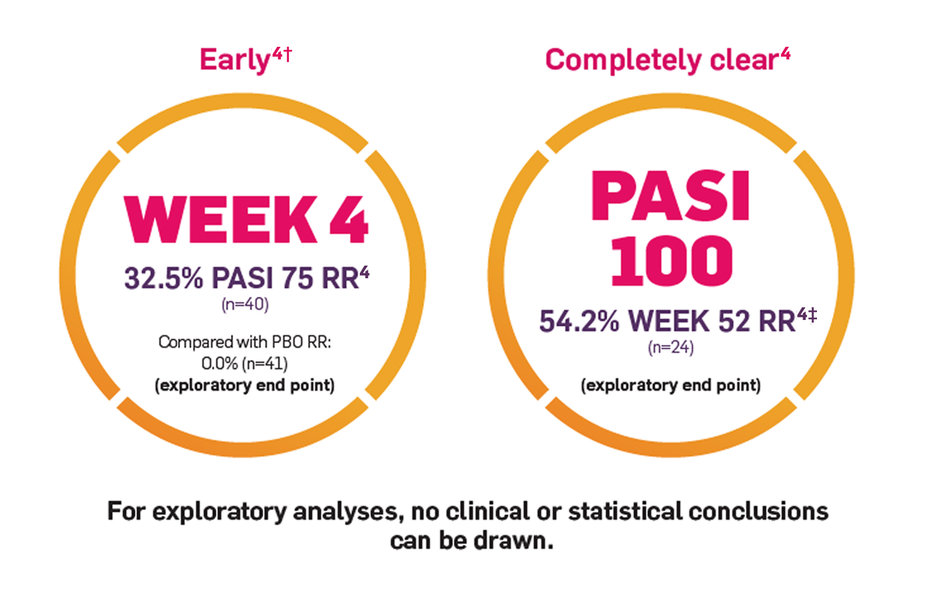
In exploratory analyses of Trial PsO6
Results observed for COSENTYX low-dose arm†
In Trial PsO6, patients were randomized into two COSENTYX arms:
Low-dose arm: 75 mg for body weight (BW) <50 kg or 150 mg for ≥50 kg2
High-dose arm: 75 mg for BW <25 kg, 150 mg for BW ≥25 kg and <50 kg (2 times the recommended dose), or 300 mg for BW ≥50 kg (2 times the recommended dose)2
†COSENTYX low-dose arm: patients weighing <50 kg received 75 mg doses. Data presented here do not account for 3 subjects from the COSENTYX high-dose arm weighing <25 kg who received COSENTYX 75 mg, based on the recommended dosing for pediatric patients <50 kg.5
‡In this study, an IRT error led to additional dosing and higher dosing of some patients (LD: n=16; HD: n=20) at Weeks 13, 14, and 15, after the primary end point (Week 12) assessment. This value represents the population not affected by the dosing error.5
In an exploratory analysis in the open-label Trial PsO7 at Week 24
2 out of 3 patients achieved PASI 1006
66.7% achieved PASI 100 in the COSENTYX low-dose arm
(75 mg or 150 mg; n=42)
No clinical or statistical conclusions can be drawn.
Trial PsO7 in pediatric patients with moderate to severe PsO
Randomized, open-label trial to assess the efficacy, long-term safety and tolerability of COSENTYX compared to historical PBO in subjects from 6 to <18 years of age with moderate to severe chronic PsO (defined by a PASI score ≥12, IGA mod 2011 score of ≥3, and BSA involvement of ≥10% at randomization).6
Patients were randomized into two COSENTYX arms in the same manner as Trial PsO6.
Coprimary end point: low-dose arm (75 mg or 150 mg) at Week 12 (n=42): 93% of patients achieved PASI 75 and 79% achieved IGA 0/1; the estimated probability of a positive treatment effect for COSENTYX vs historical placebo was 100%.6
*PBO-PASI 75 nonresponders at Week 12 were switched to either the COSENTYX low-dose or COSENTYX high-dose treatment group in the maintenance period according to the pre-assignment at their baseline randomization visit. PBO subjects who were PASI 75 responders at Week 12 had to terminate the study.4
†In this study, an IRT error led to additional dosing and higher dosing of some patients (LD: n=16; HD: n=20) at Weeks 13, 14, and 15, after the primary end point (Week 12) assessment. The number of patients in the affected and not-affected patient groups have been deemed to be too low for any differences to be considered as clinically relevant.
‡Data presented here do not account for 3 subjects from the COSENTYX high-dose arm weighing <25 kg who received COSENTYX 75 mg, based on the recommended dosing for pediatric patients <50 kg.
LD, loading dose; PASI, Psoriasis Area and Severity Index; PBO, placebo; pNRI, pure nonresponder imputation; q1w, once per week.
Definitions
AS, ankylosing spondylitis; BSA, body surface area; ERA, enthesitis-related arthritis; IGA, Investigator's Global Assessment modified 2011; LD, loading dose; nr-axSpA, non-radiographic axial spondyloarthritis; PASI, Psoriasis Area and Severity Index; PBO, placebo; pNRI, pure nonresponder imputation; PsA, psoriatic arthritis; PsO, plaque psoriasis; q1w, once per week; RR, response rate.
References
1. Data on file. AIN457A2102 Clinical Study Report. Novartis Pharmaceuticals Corp; December 2008.
2. Cosentyx. Prescribing Information. Novartis Pharmaceuticals Corp.
3. Cosentyx FDA approval history. Drugs.com. Accessed October 14, 2025. https://www.drugs.com/history/cosentyx.html
4. Data on file. CAIN457A2310 Clinical Study Report. Week 52 Analysis. Novartis Pharmaceuticals Corp; September 2019.
5. Bodemer C, Kaszuba A, Kingo K, et al. Secukinumab demonstrates high efficacy and a favourable safety profile in paediatric patients with severe chronic plaque psoriasis: 52-week results from a Phase 3 double-blind randomized, controlled trial. J Eur Acad Dermatol Venereol. 2021;35(4):938-947 and supplement.
6. Data on file. CAIN457A2311 Clinical Study Report. Week 24 Analysis. Novartis Pharmaceuticals Corp; September 2019.