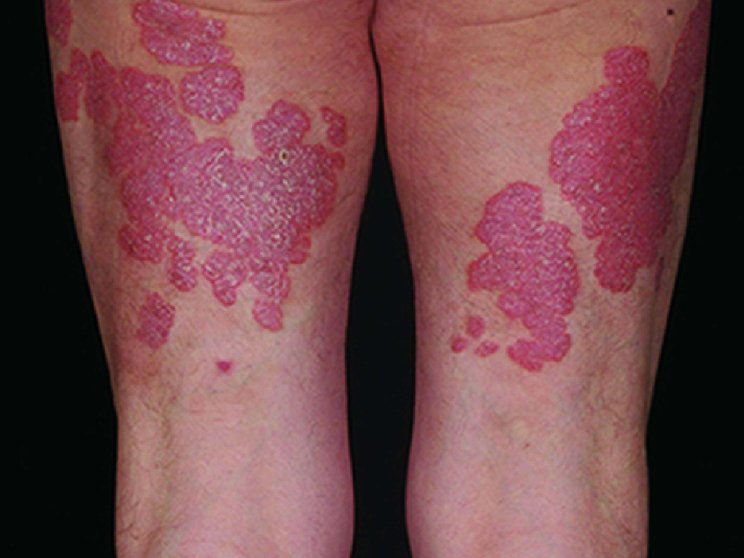
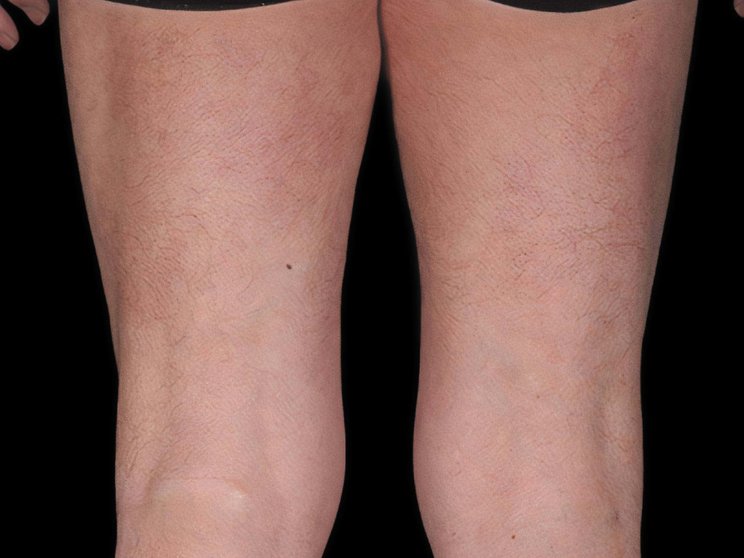
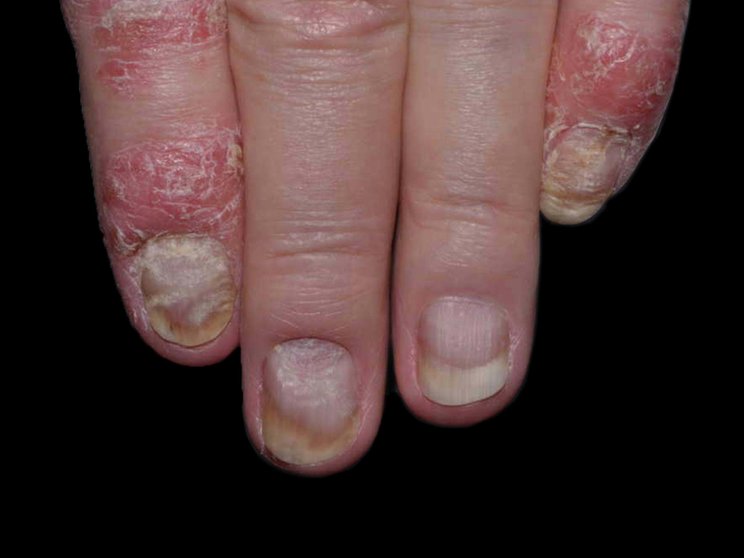
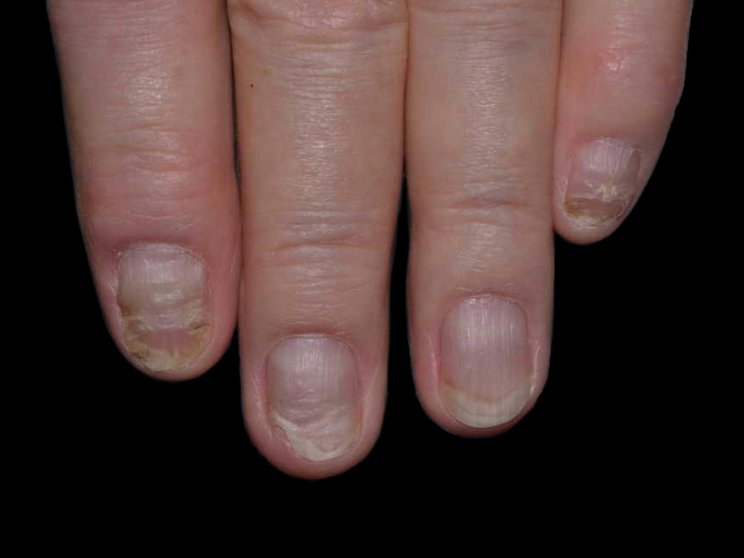
Start with skin
Complete clearance is achievable1
PASI 100 achieved by 55% of patients with the UnoReady® Pen at Week 52 in MATURE.
Observation of higher COSENTYX exposure was an exploratory end point. No clinical or statistical conclusions can be drawn
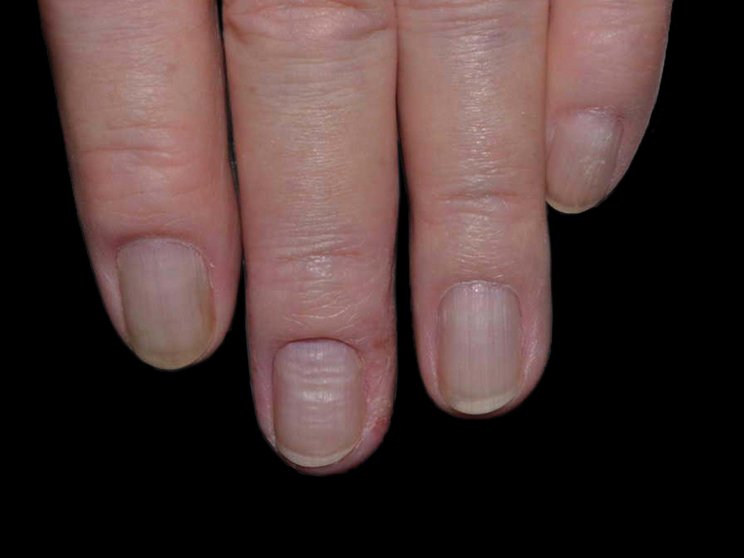
Early, strong, and sustained clearance in nail psoriasis2,3
Nail psoriasis results seen as early as Week 22*
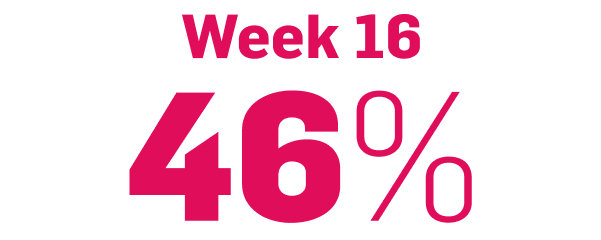
improvement in NAPSI2‡
COSENTYX 300 mg (n=66) vs 12% with placebo (n=65) (MI)
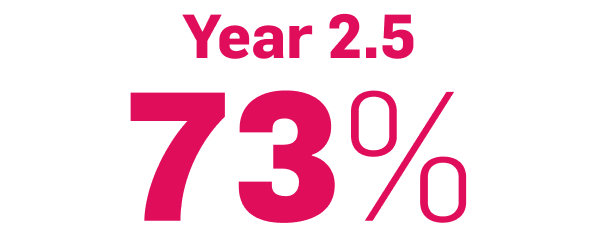
improvement in NAPSI3
COSENTYX 300 mg (n=66) (as observed)
First IL-antagonist with a dedicated trial in nail psoriasis4
NAPSI at Week 2 and through Year 2.5 was a prespecified exploratory end point. No statistical or clinical conclusions can be drawn.2
*Mean reduction in NAPSI from baseline was 5% with COSENTYX 300 mg (n=66) vs 2% with placebo (n=29).3
†Mean baseline NAPSI score was 43.2
‡Improvement was defined as mean reduction in NAPSI from baseline.2
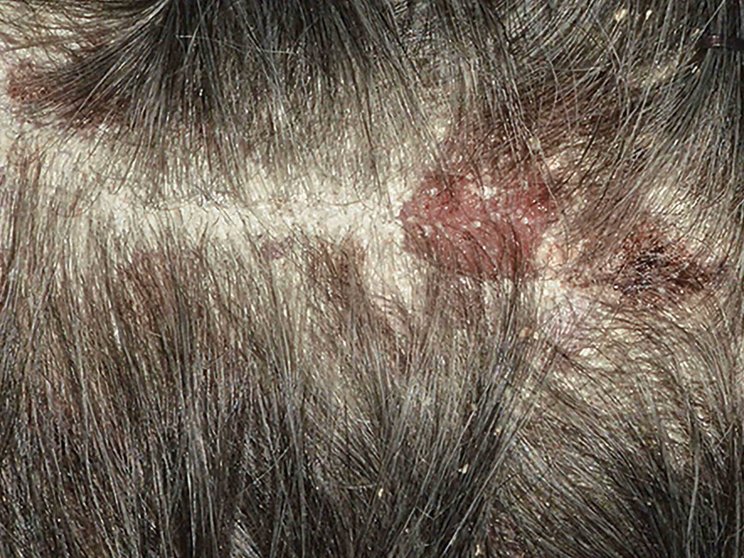
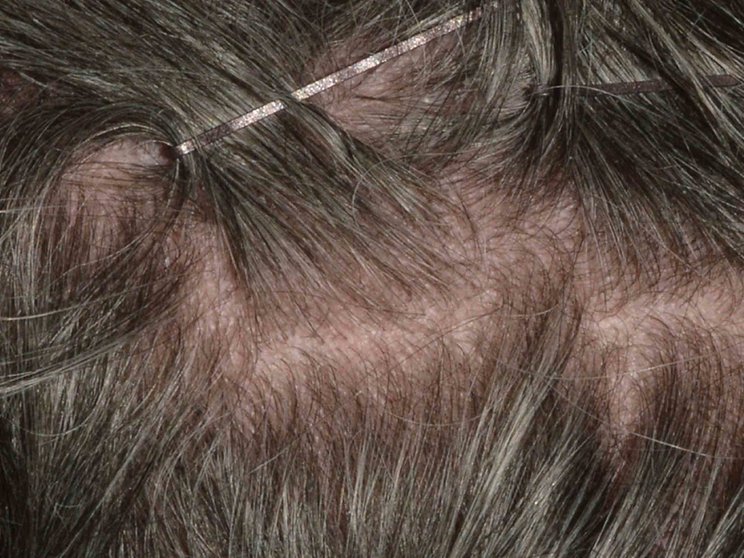
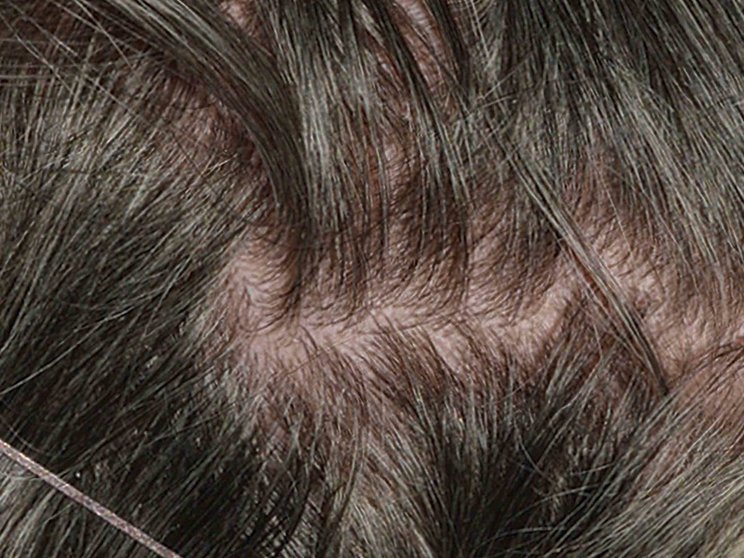
Early, strong, and sustained visible results in hard-to-treat scalp psoriasis5,6
Scalp psoriasis results seen as early as Week 35§
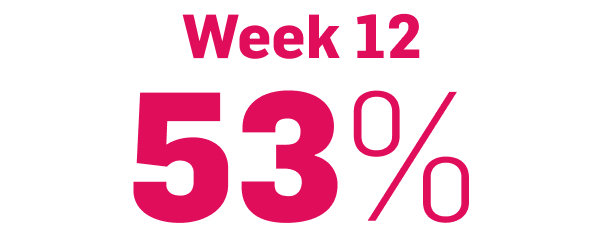
achieved PSSI 90
COSENTYX 300 mg (n=51) vs 2% with placebo (n=51) (NRI)5,6
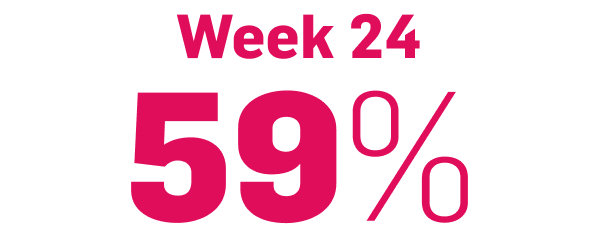
achieved PSSI 90
COSENTYX 300 mg (n=51) (NRI)5,6
First IL-antagonist with a dedicated trial in scalp psoriasis7
§12% achieved PSSI 90 with COSENTYX 300 mg vs 0% with placebo (n=51).5,6
||Mean baseline PSSI score was 33.5
Fast and long-lasting skin clearance in adult patients with PsO
Faster onset vs IL-12/23 targeting agent in CLARITY8
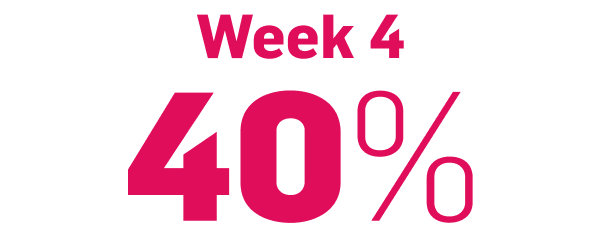
achieved PASI 75
COSENTYX 300 mg (n=550) vs 16% with ustekinumab 45/90 mg (n=552) (key secondary end point; multiple imputation, P<0.0001)
5-year skin clearance in SCULPTURE Extension9

achieved PASI 90
COSENTYX 300 mg (as observed, N=122)
75% of patients who entered the extension study at Year 1 completed the study at Year 5
COSENTYX is the first
IL-17A antagonist with
5-year PsO efficacy and safety data in a phase 3 study.10,11
Definitions
IL, interleukin; IGA, Investigator’s Global Assessment modified 2011; MI, multiple imputation; NAPSI, Nail Psoriasis Severity Index; NRI, nonresponder imputation; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, plaque psoriasis; PSSI, Psoriasis Scalp Severity Index.
References
1. Sigurgeirsson B, Browning J, Tyring S, et al. Secukinumab demonstrates efficacy, safety, and tolerability upon administration by 2 ml autoinjector in adult patients with plaque psoriasis: 52-week results from MATURE, a randomized, placebo-controlled trial. Dermatol Ther. 2022;35(3):e15285. doi:10.1111/dth.15285
2. Reich K, Sullivan J, Arenberger P, et al. Effect of secukinumab on the clinical activity and disease burden of nail psoriasis: 32-week results from the randomized placebo-controlled TRANSFIGURE trial. Br J Dermatol. 2019;181(5):954-966.
3. Reich K, Sullivan J, Arenberger P, et al. Secukinumab shows high and sustained efficacy in nail psoriasis: 2.5-year results from the randomized placebo-controlled TRANSFIGURE study. Br J Dermatol. 2021;184(3):425-436.
4. Novartis’ Cosentyx® is first biologic to show long-term efficacy in nail and palmoplantar psoriasis, which can impact up to 90% of psoriasis patients [press release]. Basel, Switzerland: Novartis; November 30, 2017. Accessed October 10, 2024. https://www.novartis.com/news/media-releases/novartis-cosentyx-first-biologic-show-long-term-efficacy-nail-and-palmoplantar-psoriasis-which-can-impact-90-psoriasis-patients
5. Bagel J, Duffin KC, Moore A, et al. The effect of secukinumab on moderate-to-severe scalp psoriasis: results of a 24-week, randomized, double-blind, placebo-controlled phase 3b study. J Am Acad Dermatol. 2017;77(4):667-674.
6. Data on file. CAIN457AUS01 Clinical Study Report. Novartis Pharmaceuticals Corp; February 2017.
7. Novartis receives FDA approval for Cosentyx® label update to include moderate to severe scalp psoriasis [press release]. Basel, Switzerland: Novartis; February 8, 2018. Accessed October 10, 2024. https://www.novartis.com/us-en/news/media-releases/novartis-receives-fda-approval-cosentyx-label-update-include-moderate-severe-scalp-psoriasis
8. Bagel J, Nia J, Hashim PW, et al. Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16-week CLARITY results). Dermatol Ther (Heidelb). 2018;8(4):571-579.
9. Bissonnette R, Luger T, Thaçi D, et al. Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol. 2018;32(9):1507-1514.
10. Data on file. CAIN457A2304E1 Clinical Study Report. Novartis Pharmaceuticals Corp; March 2018.
11. Novartis announces FDA approval for first IL-17A antagonist Cosentyx™ (secukinumab) for moderate-to-severe plaque psoriasis [press release]. Basel, Switzerland: Novartis; January 21, 2015. Accessed May 3, 2023. https://www.novartis.com/news/media-releases/novartis-announces-fda-approval-first-il-17a-antagonist-cosentyxtm-secukinumab-moderate-severe-plaque-psoriasis-patients