More ways to administer COSENTYX® subcutaneously
Please see important administration information below
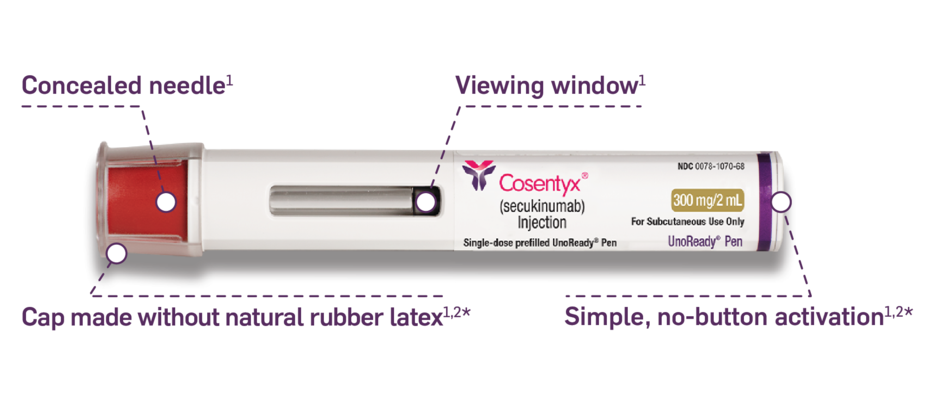
The COSENTYX you know, available in a 300-mg ALL-IN-ONE UnoReady pen1
Automatic 2-click technology so patients can hear when the injection starts and is almost finished1*
Remove from refrigerator 30 to 45 minutes before use.1
Check with your patient’s health plan to see if additional documentation is required prior to starting them on the UnoReady pen. Keep in mind that both 150-mg devices, the Sensoready pen and prefilled syringe, are available for patients taking either 150-mg or 300-mg doses.
In a study of adult patients with moderate to severe PsO
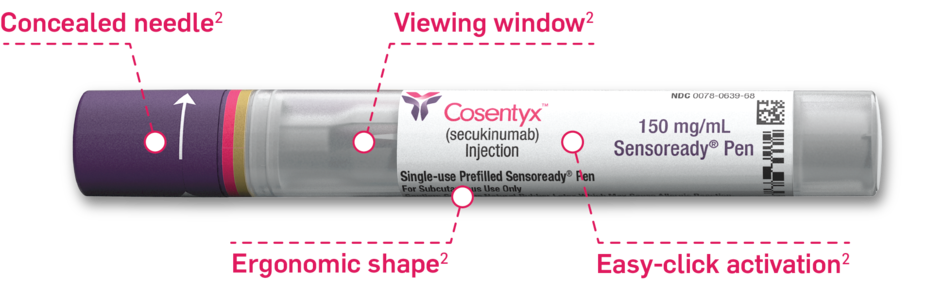
Positive injection experience with easy-to-use 150-mg Sensoready pen4‡§
Automatic 2-click technology so patients can hear when the injection starts and is almost finished1
Remove from refrigerator 15 to 30 minutes before use.1
Contains latex.1‡
In a study of adult patients with active PsA
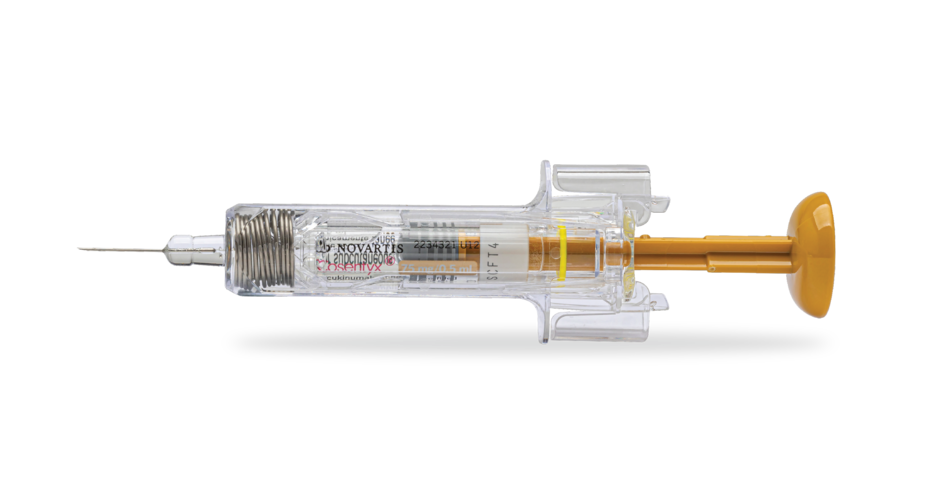
Prefilled syringe in 75-mg dose1‡¶
For children with PsA as young as 2 years and for children with ERA as young as 4 years.
Remove from the refrigerator 15 to 30 minutes before use.1
Contains latex.1‡
Also available in a 150-mg/mL prefilled syringe.
COSENTYX is intended for use under the guidance and supervision of a physician. Adult patients may self-administer COSENTYX or be injected by a caregiver after proper training in subcutaneous injection technique using the Sensoready or UnoReady pen. Pediatric patients should not self-administer COSENTYX using the prefilled syringe. An adult caregiver should prepare and inject COSENTYX after receiving training on the right way to prepare and inject COSENTYX. Inspect COSENTYX visually for particulate matter and discoloration prior to administration. Do not use if the liquid contains visible particles, is discolored or cloudy. Discard unused product.1
†n=56 out of 57 evaluable patients.3
‡The removable caps of the COSENTYX Sensoready pen and the prefilled syringes contain natural rubber latex and should not be handled by latex-sensitive individuals. The safe use of the COSENTYX Sensoready pen or prefilled syringe in latex-sensitive individuals has not been studied.1
§Feelings about injection (FL), self-confidence (CO), and satisfaction with self-injection (SA) were evaluated in 414 patients with active PsA in the FUTURE 3 study based on the Self-Injection Assessment Questionnaire. On a scale of 0 to 10, with higher score indicating more ease of use and greater satisfaction, the mean scores at baseline were 8.2 FL, 6.5 CO, and 6.8 SA. At Week 2, the mean scores were 8.8 FL, 7.8 CO, and 8.4 SA.4
||N=414.4
¶May be administered with or without methotrexate for children with PsA.1
Definitions
ERA, enthesitis-related arthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis.
References
1. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
2. Paul C, Lacour J-P, Tedremets L, et al; for the JUNCTURE Study Group. Efficacy, safety and usability of secukinumab administration by autoinjector/pen in psoriasis: a randomized, controlled trial (JUNCTURE).
J Eur Acad Dermatol Venereol. 2015;29(6):1082-1090.
3. Data on file. CAIN457A2325 (MATURE): Clinical Study Report. Novartis Pharmaceuticals Corp; August 2020.
4. Nash P, Mease PJ, McInnes IB, et al; on behalf of the FUTURE 3 study group. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3). Arthritis Res Ther. 2018;20(1):47.