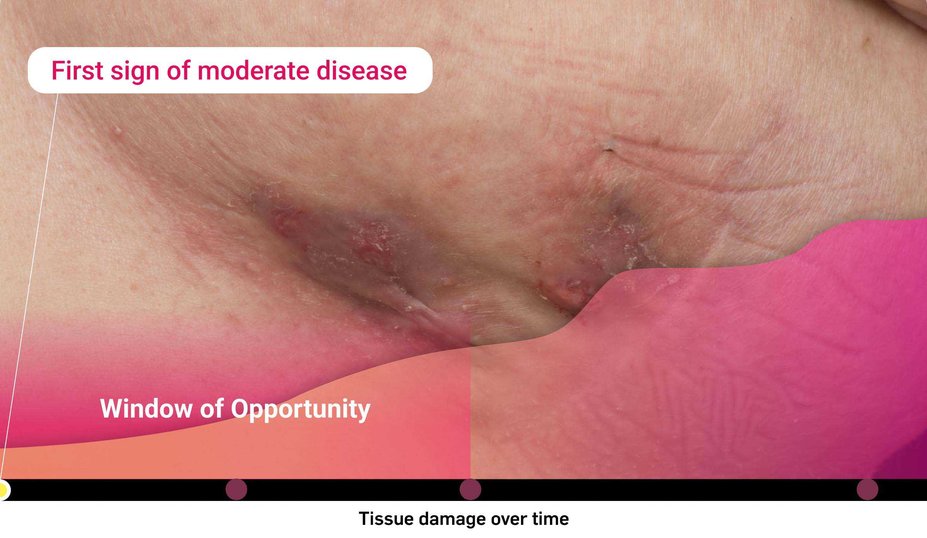
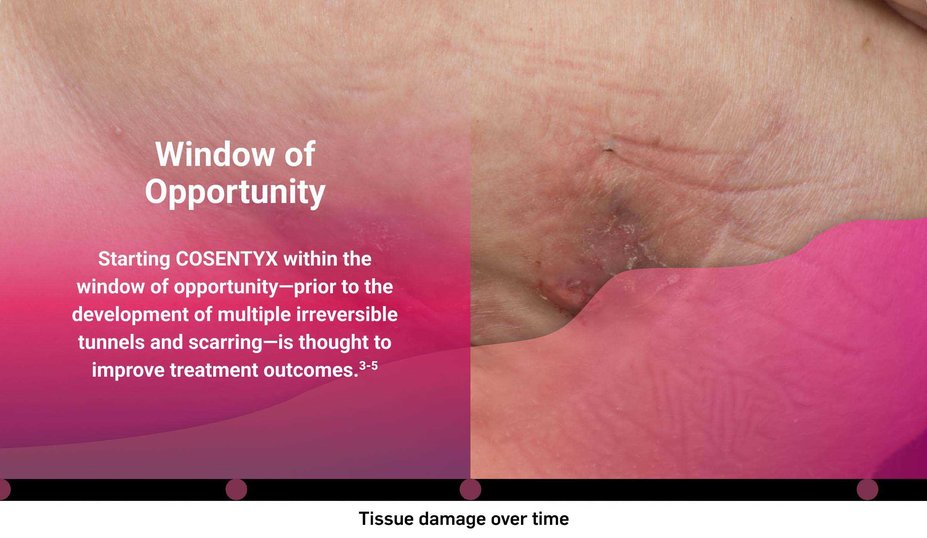
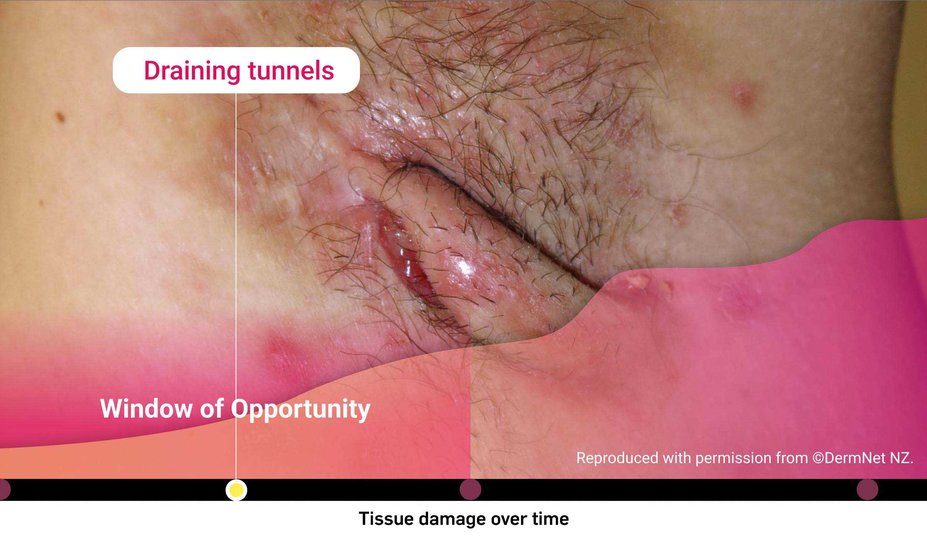
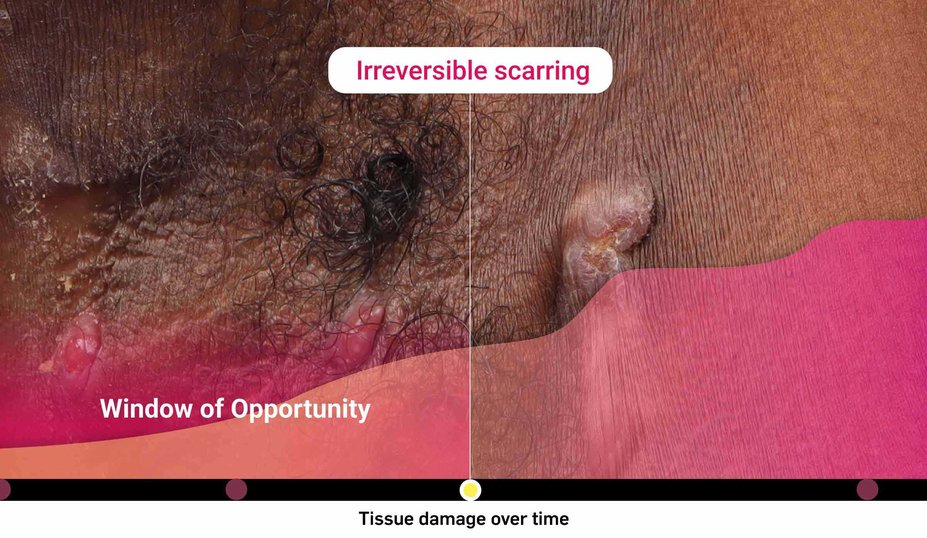
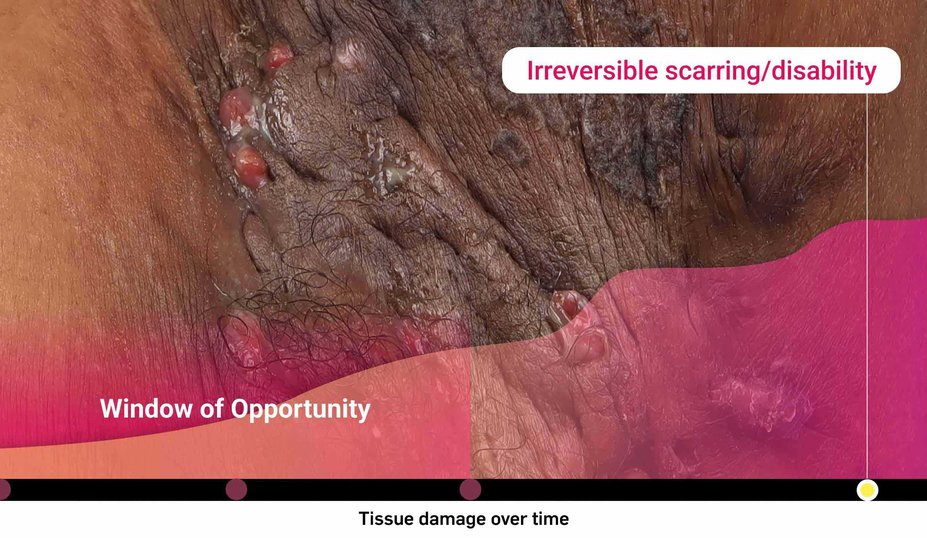
What is the risk of waiting too long for biologic intervention?
Could HS be more severe than it appears?
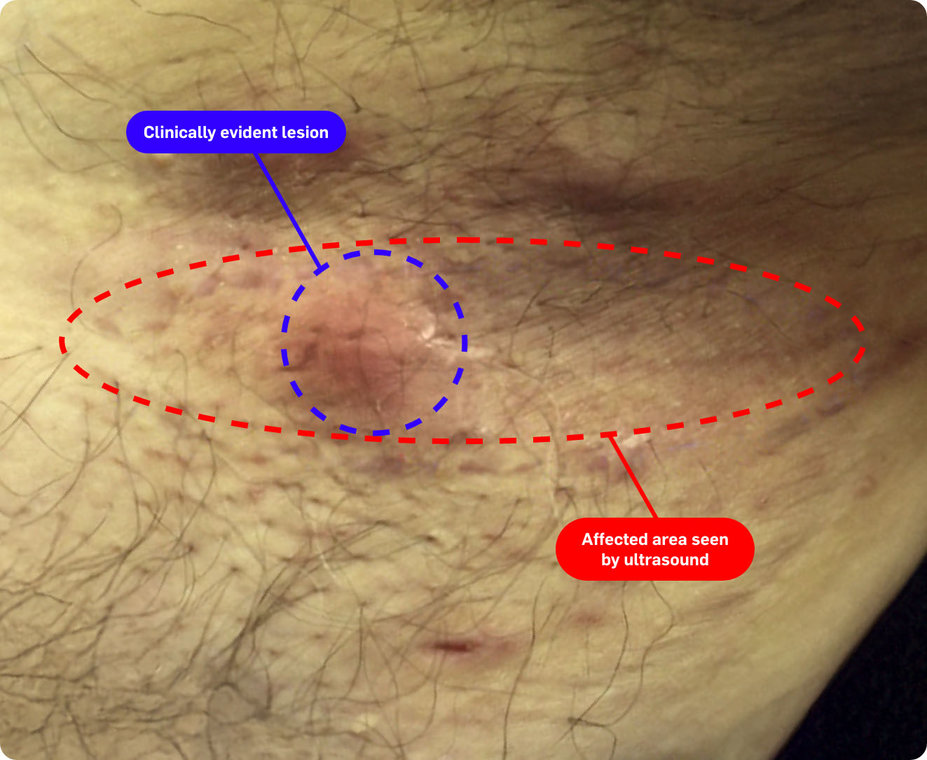
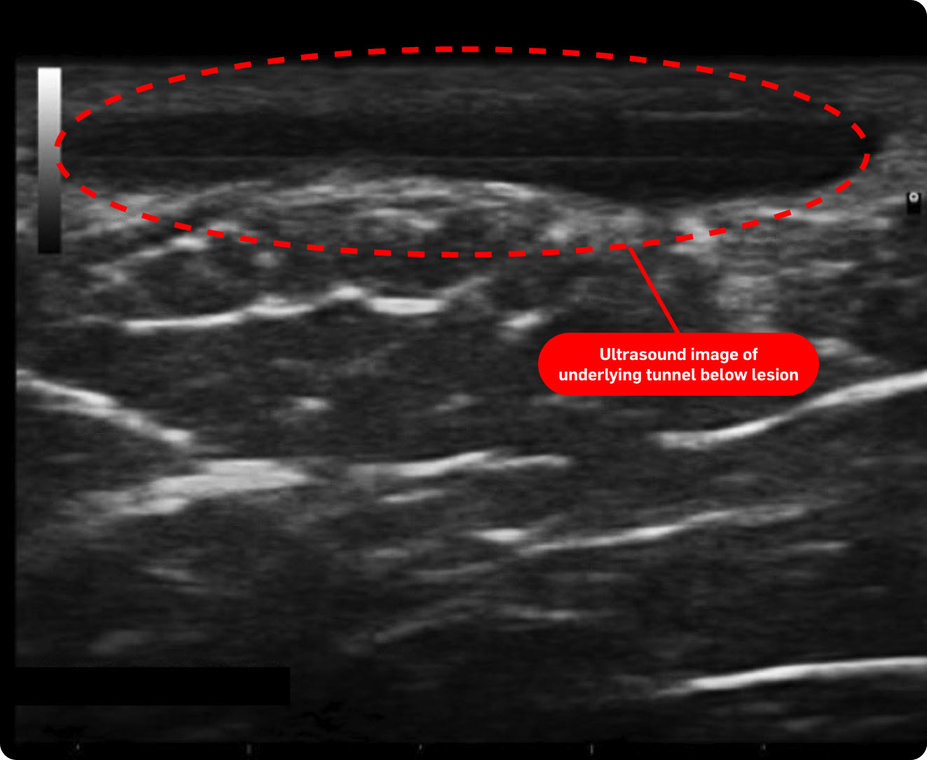
In one study, nearly 45% of patients were reclassified to be moderate or severe* after an ultrasound exam (n=38)1†
Consider both the seen and unseen signs of HS when assessing disease severity1,2

HS takes a heavy toll on patients’ emotional and
mental well-being
~97% of patients experience physical pain.6
~66% of patients experience depression.7
>2x the risk of completed suicide in patients with HS compared with the general population.8
The impacts of HS are not limited only to those listed above.
Refer a Patient
Locate a dermatologist who specializes in the treatment of HS.
*As measured by patients going from Hurley Stage I to II or III.1
†Based on Spanish diagnostic study of adult patients diagnosed with HS (N=143). The number of patients who may have been reclassified to a lower Hurley stage was not reported/noted by the study. The mean number of fistulas on ultrasound compared to clinical exam was not statistically significant.1
‡Images are from a 2015 Martorell study, not the same study as the data presented above.2
Definition
HS, hidradenitis suppurativa.
References
1. Martorell A, Alfageme Roldán F, Vilarrasa Rull E, et al. Ultrasound as a diagnostic and management tool in hidradenitis suppurativa patients: a multicentre study. J Eur Acad Dermatol Venereol. 2019;33(11):2137-2142.
2. Martorell A, García-Martínez FJ, Jiménez-Gallo D, et al. An Update on Hidradenitis Suppurativa (Part I): Epidemiology, Clinical Aspects, and Definition of Disease Severity. Actas Dermosifiliogr. 2015;106(9):703-715.
3. Martorell A, Giovanardi G, Gomez-Palencia P, Sanz-Motiva V. Defining fistular patterns in hidradenitis suppurativa: impact on the management. Dermatol Surg. 2019;45(10):1237-1244.
4. Shih T, De D, Daveluy SD, et al. Real-world considerations of candidacy for biologics in hidradenitis suppurativa. Am J Clin Dermatol. 2022;23(6):749-753.
5. van der Zee HH, Zouboulis CC, Reguiai Z, et al. The impact of lesion type on clinical response with secukinumab in patients with moderate to severe hidradenitis suppurativa: A post hoc analysis of the pooled data from SUNSHINE and SUNRISE phase 3 trials. Poster presented at 33rd European Academy of Dermatology and Venereology (EADV) Congress, September 25-28, 2024; Amsterdam, The Netherlands. Poster P0186.
6. Matusiak Ł, Szczȩch J, Kaaz K, Lelonek E, Szepietowski JC. Clinical characteristics of pruritus and pain in patients with hidradenitis suppurativa. Acta Derm Venereol. 2018;98(2):191-194.
7. McKenzie SA, Harview CL, Truong AK, et al. Physical symptoms and psychosocial problems associated with hidradenitis suppurativa: correlation with Hurley stage. Dermatol Online J. 2020;26(9):13030/qt4rm8w7kn.
8. Thorlacius L, Cohen AD, Gislason GH, Jemec GBE, Egeberg A. Increased suicide risk in patients with hidradenitis suppurativa. J Invest Dermatol. 2018;138(1):52-57.