COSENTYX®: The first FDA-approved IL-17A inhibitor1-3
COSENTYX selectively targets IL-17A1
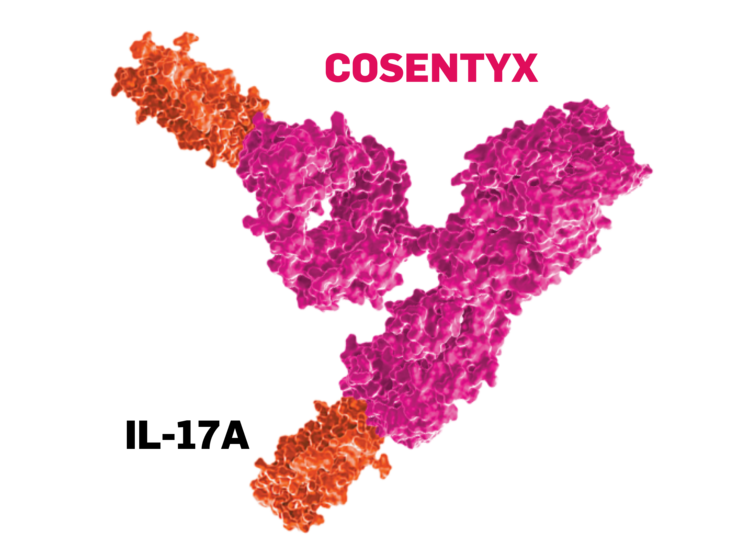
Artistic representation of the monoclonal antibody–cytokine interaction.
Image not drawn to scale.
- COSENTYX blocks IL-17A from both adaptive and innate immune sources4,5
- COSENTYX is fully human1
IL-17A plays a key role in the pathogenesis of PsO, PsA, AS, and nr-axSpA6
Image not drawn to scale.
Elevated levels have been found in:
Psoriatic plaques1
The blood of patients with PsA, AS, and nr-axSpA
Increased numbers of IL-17A–producing lymphocytes have also been found in patients with nr-axSpA1
IL-17A is a proinflammatory cytokine. Overproduction of proinflammatory cytokines, such as IL-17A, may contribute to signs and symptoms seen in patients with PsA, AS, and nr-axSpA7-9
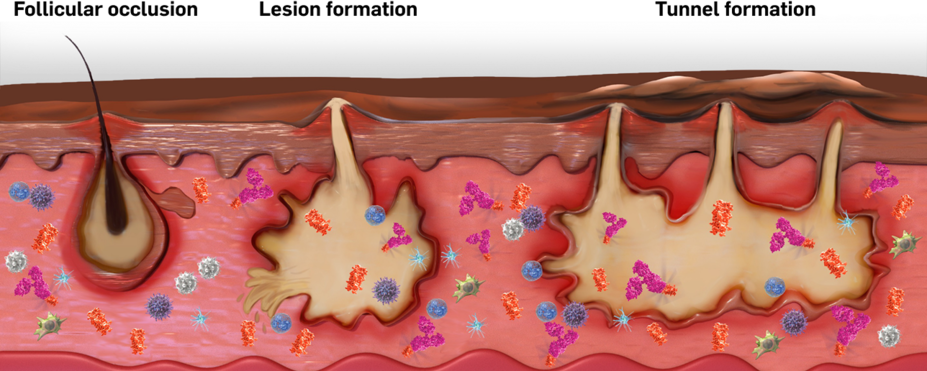
COSENTYX, a fully human biologic, selectively targets a key pathway of HS inflammation1*
Artistic representation of the monoclonal antibody–cytokine interaction.
IL-17–producing cells and IL-17A have been found in the serum and skin lesions of patients with HS.10,11
IL-17A is one of several key cytokines thought to play a role in inflammation in HS.12
Definitions
AS, ankylosing spondylitis; FDA, US Food and Drug Administration; HS, hidradenitis suppurativa; IL, interleukin; MOA, mechanism of action; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis.
References
1. Cosentyx. Prescribing information. Novartis Pharmaceuticals Corp.
2. Taltz. Prescribing information. Eli Lilly & Co.
3. Siliq. Prescribing information. Bausch Health US LLC.
4. Noack M, Beringer A, Miossec P. Additive or synergistic interactions between IL-17A or IL-17F and TNF or IL-1β depend on the cell type. Front Immunol. 2019;10:1726.
5. Kolbinger F, Di Padova F, Deodhar A, et al. Secukinumab for the treatment of psoriasis, psoriatic arthritis, and axial spondyloarthritis: physical and pharmacological properties underlie the observed clinical efficacy and safety. Pharmacol Ther. 2022;229:107925. doi:10.1016/j.pharmthera.2021.107925
6. Tsukazaki H, Kaito T. The role of the IL-23/IL-17 pathway in the pathogenesis of spondyloarthritis. Int J Mol Sci. 2020;21(17):6401.
7. Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763-776.
8. Menter A, Krueger GG, Paek SY, Kivelevitch D, Adamopoulos IE, Langley RG. Interleukin-17 and interleukin-23: a narrative review of mechanisms of action in psoriasis and associated comorbidities. Dermatol Ther (Heidelb). 2021;11(2):385-400.
9. Paine A, Ritchlin C. Targeting the interleukin-23/17 axis in axial spondyloarthritis. Curr Opin Rheumatol. 2016;28(4):359-367.
10. Kelly G, Hughes R, McGarry T, et al. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br J Dermatol. 2015;173(6):1431-1439.
11. Matusiak Ł, Szczęch J, Bieniek A, Nowicka-Suszko D, Szepietowski JC. Increased interleukin (IL)-17 serum levels in patients with hidradenitis suppurativa: implications for treatment with anti-IL-17 agents. J Am Acad Dermatol. 2017;76(4):670-675.
12. Sabat R, Jemec GBE, Matusiak Ł, Kimball AB, Prens E, Wolk K. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18.